Keul-o-test®
RSV-Adenovirus Respiratory
KGST312
A rapid, one step test for the qualitative detection of RSV and Adenovirus antigens from human nasopharyngeal specimens (swab, nasopharyngeal wash and aspirate).
For professional in vitro diagnostic use only.
Intended Use
The RSV-Adenovirus Respiratory Device test is a rapid chromatographic immunoassay for the qualitative detection of RSV and Adenovirus antigens in human nasopharyngeal specimens to aid in the diagnosis of RSV and Adenovirus respiratory infection.
Synthesis
Although a wide variety of viral agents are capable of causing lower respiratory tract infections in children and adults, influenza A & B; respiratory syncytial virus (RSV); parainfluenza viruses 1, 2, and 3; and adenovirus are the most common. Of these, influenza A & B and RSV are the most important causes of medically attended acute respiratory illness. In addition to sharing a similar seasonal prevalence, it is important to remain cognizant that influenza A & B and RSV share overlapping clinical features and infection potential for certain high-risk patient groups (e.g., extremes of age, underlying cardiopulmonary disease and immunosuppression). Symptoms of respiratory illness caused by adenovirus infection range from the common cold syndrome to pneumonia, croup, and bronchitis.
Principle
The RSV-Adenovirus Respiratory Device is a qualitative lateral flow immunoassay for the detection of RSV and Adenovirus Respiratory antigen in human nasopharyngeal samples. The membrane is pre-coated with monoclonal antibodies against RSV and Adenovirus antigens on the test line region. During testing, the sample reacts with the particles coated with anti-RSV antibodies and anti-Adenovirus antibodies which were pre-dried on the test strip. The mixture moves upward on the membrane by capillary action. In the case of a positive result the specific antibodies present on the membrane will react with the mixture conjugate and generate one (RSV or Adenovirus) or two (RSV and Adenovirus) coloured test lines. A green coloured band always appears in the control line and serves as verification that sufficient volume was added, that proper flow was obtained and as an internal control for the reagents.
Precautions
- For professional in vitro diagnostic use only.
- Do not use after expiration date.
- The test should remain in the sealed pouch until use.
- Do not use the test if pouch is damaged.
- Follow Good Laboratory Practices, wear protective clothing, use disposal gloves, do not eat, drink or smoke in the area.
- All the specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
- The test should be discarded in a proper biohazard container after testing.
- The test must be carried out within 2 hours of opening the sealed bag.
Storage and stability
Store as packaged in the sealed pouch either at refrigerated or room temperature (2-30ºC/36-86ºF). The test is stable through the expiration date printed on the sealed pouch. The test must remain in the sealed pouch until use. Do not freeze.
Materials provided
- Devices
- Instructions for use
- Diluent B (sample diluent)
Materials required but not provided
- Specimen collection container
- Disposable gloves and timer
- Sterile swabs
- Plastic pipettes
- Testing tubes or vials
Specimen collection and preparation
Nasopharyngeal swab method:
- Bend shaft to follow curve of nasopharynx
- Insert swab through nostril to posterior nasopharynx.
- Rotate swab a few times to obtain infected cells
- For an optimal sample, repeat procedure using other nostril
Nasopharyngeal aspirate method (suction apparatus, sterile suction catheter):
- Instill several drops of solution saline into each nostril
- Place catheter through nostril to posterior nasopharynx
- Apply gentle suction. Using rotating motion, slowly withdraw catheter
- For an optimal sample, repeat procedure using other nostril
Send specimen to lab immediately (testing sensitivity decrease over time)
Cool specimen to 2º-4ºC (36º-40ºF) during storage and transport.
Procedures
Allow the tests, samples and buffers to reach to room temperature (15-30ºC/59-86ºF) prior to testing. Do not open pouches until ready to perform the assay.
To process the collected nasopharyngeal wash or aspirate samples (see illustration 1):
Use a separate pipette and testing tube for each sample. Add the nasopharyngeal wash or aspirate sample (6 drops or 300uL) in a testing tube or vial. Add the diluent B (3 drops or 150uL) and mix. Remove the RSV-Adenovirus Respiratory Device from its sealed pouch and use it as soon as possible. Use a separate device for each sample. Dispense 100uL into the specimen well (S). Start the timer. Read the result at 10 minutes after dispensing the sample.
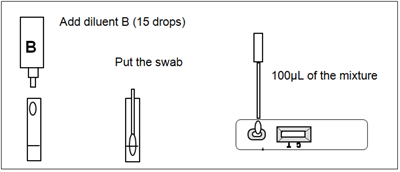
To process the collected nasopharyngeal swab (see illustration 2):
Use a separate testing tube or vial for each sample (swab). Add the diluent B (15 drops or 500uL) into the testing tube or vial, put the nasopharyngeal swab, mix and extract as much liquid possible from the swab. Remove the RSV-Adenovirus Respiratory Device from its sealed pouch and use it as soon as possible. Use a separate device for each sample. Dispense exactly 100uL into the specimen well (S). Start the timer. Read the result at 10 minutes after dispensing the sample.
Illustration 1
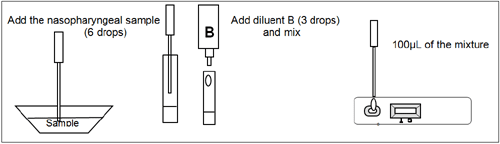
Illustration 2
Interpretation of results
Illustration 3

POSITIVE:
RSV positive: Two lines appears across the central window, in the result line region (red test line marked with the letter T) and in the control line region (green control line marked with the letter C).
Adenovirus positive: Two lines appears across the central window, in the result line region (blue test line marked with the letter T) and in the control line region (green control line marked with the letter C).
RSV-Adenovirus positive: Three lines appears across the central window, in the result line region two lines (red test line and blue test line marked with the letter T) and in the control line region (green control line marked with the letter C).
NEGATIVE: Only one green band appears across the control line region marked with the letter C (control line).
INVALID: A total absence of the green control coloured band regardless the appearance or not of the red and blue test lines. Note: Insufficient specimen volume, incorrect procedural techniques or deterioration of the reagents are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the test kit and contact you local distributor.
Notes on the interpretation of results
The intensity of the red/blue coloured band in the result line region (T) will vary depending on the concentration of antigens in the specimen. However, neither the quantitative value, nor the rate of increase in antigens can be determined by this qualitative test.
Quality control
Internal procedural controls are included in the test:
- A green line appearing in the control line region (C). It confirms sufficient specimen volume and correct procedural technique.
Limitations
- RSV-Adenovirus Respiratory Device will only indicate the presence of RSV and/or Adenovirus in the specimen (qualitative detection) and should be used for the detection of RSV or Adenovirus antigens in nasopharyngeal specimens only (from swab, aspirate or wash). Neither the quantitative value nor the rate of increase in antigens concentration can be determined by this test.
- If the test result is negative and clinical symptoms persist, additional testing using other clinical methods is recommended. A negative result does not at any time preclude the possibility of RSV or Adenovirus infection.
- This test provides a presumptive diagnosis of RSV and Adenovirus respiratory infections. All results must be interpreted together with other clinical information and laboratory findings available to the physician.
Expected values
RSV is generally considered the most frequent cause of pneumonia, bronchiolitis, and tracheobronchitis among infants and young children, it is now known to be the etiologic cause in 14-27% of cases of pneumonia in the elderly during the winter season.
Everyone is at risk of adenovirus infection, but patients with weak immune systems or with underlying respiratory or cardiac disease are most at risk for severe complications from any respiratory infection, including adenovirus infections.
Performance characteristics
Sensitivity and specificity
Different virus extract dilutions were tested directly in the sample diluent or spiked in a negative nasal specimen in accordance with the kit instructions.
The detection of RSV showed >95% of sensitivity compared with another commercial rapid test and showed >99% of specificity compared with the commercial rapid test.
The RSV-Adenovirus Respiratory Device was highly specific (>99%) to detect Adenovirus and also sensitive (>99%) compared with the results of an immunofluorescence assay (Remel).
Cross-Reactivity
It was performed an evaluation to determine the cross reactivity of RSV-Adenovirus Respiratory Device. There is not cross reactivity with common respiratory pathogens, other organisms and substances occasionally present in nasopharyngeal samples:
References
- BARENFANGER et al., „Clinical and Financial Benefits of Rapid Detection of Respiratory Viruses: an Outcomes Study“. Journal of Clinical Microbiology. August 2000, Vol 38 No 8, p. 2824-2828.
Qualitätssicherung und Vorkommnisse
Sollten Sie den Eindruck eines Qualitätsmangels haben oder unklare oder ihrerseits falsch-positive oder falsch-negative Ergebnisse erhalten, bitten wir Sie, die betreffende Patientenprobe zurückzustellen und für einen Abruf für uns bereitzuhalten.
Bitte informieren Sie uns umgehend. Sie helfen uns dadurch die Sicherheit der Produkte und damit die Qualität zu gewährleisten.
 |
Hersteller: |

Günter Keul GmbH
Von-Langen-Weg 10
D-48565 Steinfurt
Tel.: 02551/2097 Fax.: 02551/80883
Web: www.keul.de |
|