| |
Keul-o-test®
FOB-Transferrin
KGST308
A rapid and one step test for the qualitative detection of human haemoglobin (hHb) and transferrin (hTf) in human faeces. For professional in vitro diagnostic use only.
Intended Use
The FOB-Transferrin Device is a rapid chromatographic immunoassay (non-invasive assay) for the qualitative detection of human haemoglobin and transferrin in human faeces specimens, which might be useful for the diagnosis of bleeding gastrointestinal disorders.
Synthesis
Colorectal cancer is cancer that occurs in the colon or rectum, and affects both men and women of all racial and ethnic groups, and is most often found in people aged 50 years or older. For men, colorectal cancer is the third most common cancer after prostate and lung cancers. For women, colorectal cancer is the third most common cancer after breast and lung cancers.
Fecal occult blood should be an important indicator in the diagnostic evaluation of patients with suspected gastrointestinal bleeding of any etiology, not just as an indication of colorectal cancer. The presence of human haemoglobin in faeces is inadequate as a screening test for stomach cancer (upper gastrointestinal disorders), because of human haemoglobin derived from the upper digestive tract is broken down in the intestinal tract (the antigenicity is lost).
Detection of fecal transferrin, which is more stable in stool than haemoglobin, provides an alternative way of diagnosing the disease in the upper digestive tract.
Blood in the stool may be the only symptom of cancer, but not all blood in the stool is caused by cancer. Other conditions that can cause blood in the stool include: Haemorrhoids, Anal fissures, Colon polyps, Peptic ulcers, Ulcerative colitis. Gastroesophageal reflux disease (GERD). Crohn’s disease, use of non-steroidal anti-inflammatory drugs (NSAIDs).
Principle
The FOB-Transferrin Device is a qualitative lateral flow immunoassay for the detection of human haemoglobin and human transferrin in faeces samples. The membrane is pre-coated with monoclonal antibodies against human haemoglobin and human transferrin on the test lines region. During testing, the sample reacts with the particles coated with anti-human haemoglobin and anti-human transferrin antibodies which were pre-dried on the test strip. The mixture moves upward on the membrane by capillary action. In the case of a positive result the specific antibodies present on the membrane will react with the mixture conjugate and generate coloured lines. A green coloured band always appears in the control line and serves as verification that sufficient volume was added, that proper flow was obtained and as an internal control for the reagents.
Precautions
- For professional in vitro diagnostic use only.
- Do not use after expiration date.
- The test should remain in the sealed pouch until use.
- Do not use the test if pouch is damaged.
- Follow Good Laboratory Practices, wear protective clothing, use disposal gloves, do not eat, drink or smoke in the area.
- All the specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
- The test should be discarded in a proper biohazard container after testing.
- The test must be carried out within 2 hours of opening the sealed bag.
Storage and stability
Store as packaged in the sealed pouch either at refrigerated or room temperature (2-30ºC/36-86ºF). The test is stable through the expiration date printed on the sealed pouch. The test must remain in the sealed pouch until use. Do not freeze.
Materials provided
- Devices
- Instructions for use
- Specimen collection vial with buffer
Materials required but not provided
- Specimen collection container
- Disposable gloves
- Timer
Specimen collection and preparation
Collect sufficient quantity of faeces (1-2 g or mL for liquid sample). Stool samples should be collected in clean and dry containers (no preservatives or transport media). The samples can be stored in the refrigerator (2-4ºC/36-40ºF) for 1-2 days prior to testing. For longer storage the specimen must be kept frozen at -20ºC/4ºF. In this case, the sample will be totally thawed, and brought to room temperature before testing.
Procedures
To process the collected stool samples (see illustration 1):
Use a separate specimen collection vial for each sample. Unscrew the cap of the vial and introduce the stick three times into the faecal specimen to pick up the sample (approx. 15mg). Close the vial with the buffer and stool sample. Shake the vial in order to assure good sample dispersion. For liquid stool samples, aspirate the faecal specimen with a dropper and add approx. 15μL into the specimen collection vial with buffer.
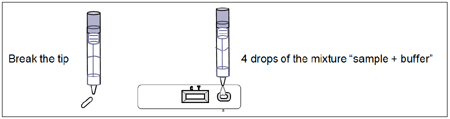
Test Procedure (see illustration 2)
Allow the tests, stool samples and buffer to reach to room temperature (15-30ºC/59-86ºF) prior to testing. Do not open the pouch until ready to perform the assay.
- Remove the FOB-Transferrin Device from its sealed pouch and use it as soon as possible.
- Shake the specimen collection vial to assure a good sample dispersion. Break the tip of the vial.
- Use a separate device for each sample. Dispense 4 drops into the specimen well (S). Start the timer.
- Read the result at 10 minutes after dispensing the sample.
Illustration 1
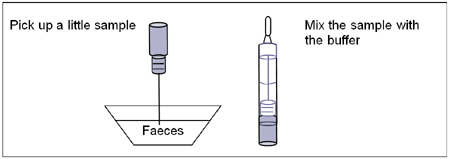
Illustration 2
Interpretation of results
Illustration 3

Tf positive: Two lines appears across the central window, in the result line region (red test line marked with the letter T) and in the control line region (green control line marked with the letter C). A Transferrin (Tf) positive result could be indicative of upper gastrointestinal bleeding.
Hb positive: Two lines appears across the central window, in the result line region (blue test line marked with the letter T) and in the control line region (green control line marked with the letter C). The result Haemoglobin (Hb) positive is indicative of little blood in faeces; transferrin exists in only trace amounts in blood and could be not detected.
Tf-Hb positive: Three lines appears across the central window, in the result line region two lines (red test line and blue test line marked with the letter T) and in the control line region (green control line marked with the letter C). Transferrin (Tf) and Haemoglobin (Hb) positive result could be indicative of lower gastrointestinal bleeding disorders.
NEGATIVE: Only one green band appears across the control line region marked with the letter C (control line). Not occult blood in faeces.
INVALID: A total absence of the green control coloured band regardless the appearance or not of the red test line. Note: Insufficient specimen volume, incorrect procedural techniques or deterioration of the reagents are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the test kit and contact you local distributor.
Notes on the interpretation of results
The intensity of the blue or red coloured band in the result line region (T) will vary depending on the concentration of human haemoglobin or human transferrin in the specimen. However, neither the quantitative value, nor the rate of increase in haemoglobin/transferrin can be determined by this qualitative test.
Quality control
Internal procedural controls are included in the test:
- A green line appearing in the control line region (C). It confirms sufficient specimen volume and correct procedural technique.
Limitations
- FOB-Transferrin will only indicate the presence of human haemoglobin or/and transferrin in the specimen (qualitative detection) and should be used for the detection of haemoglobin and transferrin in faeces specimens only. Neither the quantitative value nor the rate of increase in haemoglobin or transferrin concentration can be determined by this test.
- An excess of sample could cause wrong results (brown bands appear). Dilute the sample with the buffer and repeat the test.
- Some stool samples can decrease the intensity of the control line.
- In the case of patients have bleeding haemorrhoids, blood in urine, strained during bowel movement or women during their menstrual period, should be not collect samples.
- Positive results confirm the presence of occult blood in faecal samples; nevertheless, it can be due to several causes, besides colorectal bleeding, such as haemorrhoids, anal fissures, colon polyps, peptic ulcers, ulcerative colitis. gastroesophageal reflux disease (GERD), Crohn’s disease. Positive results should be followed up with additional diagnostic procedures by a physician to determine the exact cause and source of the blood in the stool. Endoscopy is the method of choice in diagnosing the cause of upper and lower gastrointestinal bleeding.
- Negative results do not exclude bleeding since some polyps and colorectal cancers may bleed intermittently or not at all during certain stages of the disease. Additionally, blood may not be uniformly distributed in stool samples
Expected values
Common causes of Upper GI bleeding: duodenal ulcer (20-30%), gastric or duodenal erosions (20-30%), varices (15-20%), gastric ulcer (10-20%), erosive esophagitis (5-10%), angioma (5-10%), arteriovenous malformation (<5%), gastrointestinal stromal tumours.
Common causes of Lower GI bleeding (percentages vary with the age group sampled): anal fissures, angiodysplasia (vascular ectasia), colitis (radiation, ischemic, infectious), colonic carcinoma, colonic polyps, diverticular disease, inflammatory bowel disease: ulcerative, proctitis/colitis, Crohn’s disease, internal haemorrhoids.
Performance characteristics
Sensitivity and Specificity
The detection limit of FOB-Transferrin Device is 50ng/mL for haemoglobin and 4ng/mL for transferrin.
FOB-Transferrin Device was highly specific (>99%) and also highly sensitive (>99%) compared with the results of that guaiac assay.
The detection of hHb with FOB-Transferrin Device showed >99% of sensitivity and >99% of specificity compared with others commercial rapid tests (ImmunoTech OcculTech and Human Hexagon OBTI).
Cross-Reactivity and interferences
It was performed an evaluation to determine the cross reactivity and interferences of FOB-Transferrin Device. There is not cross reactivity with common gastrointestinal pathogens, other organisms and substances occasionally present in feces.
- Rotavirus
- Astrovirus
- Adenovirus
- Eschericha coli
- Campylobacter
- Giardia lamblia
- Lactoferrin
No special diet is recommended prior to testing. There are not interferences with any foods (vitamin C, broccoli, carrots…) and supplements (iron).
References
- WALKER C.W., „Fecal occult blood tests reduce colorectal cancer mortality.“, Am Fam Physician. 2007 Jun 1;75(11):1652-3.
- CHIEN-HUA CHIANG, et al. «A comparative study of three fecal occult blood tests in upper gastointestinal bleeding»; Kaohsiung J. Med. Sci May 2006, Vol 22, No 5: 223-228
- HIROFUMI MIYOSHI, et al. «Accuracy of Detection of Colorectal Neoplasia using an Immunochemical Occult Blood Test in Symptomatic Referred Patients: Comparison of Retrospective and Prospective Studies. Internal Medicine Sept. 2000 Vol. 39, No. 9: 701-706.
Qualitätssicherung und Vorkommnisse
Sollten Sie den Eindruck eines Qualitätsmangels haben oder unklare oder ihrerseits falsch-positive oder falsch-negative Ergebnisse erhalten, bitten wir Sie, die betreffende Patientenprobe zurückzustellen und für einen Abruf für uns bereitzuhalten.
Bitte informieren Sie uns umgehend. Sie helfen uns dadurch die Sicherheit der Produkte und damit die Qualität zu gewährleisten.
 |
Hersteller: |

Günter Keul GmbH
Von-Langen-Weg 10
D-48565 Steinfurt
Tel.: 02551/2097 Fax.: 02551/80883
Web: www.keul.de |
|
|