| |
Keul-o-test
Clostridium Difficile B
KGST202
DIMDI Reg.-Nr.: DE/CA22/1116-133-IVD
Immunochromatographic rapid test for the detection of Clostridium Difficile Toxin B in feces

I. PRINCIPLE
Clostridium difficile is a major cause of antibiotic-associated diarrhea and pseudomembranous colitis (1). It is now one of the most commonly detected pathogens and an important cause of nosocomial infections in hospitals and nursing homes (2, 3).
The organism has been isolated from diverse natural habitats, including soils, hay, sand, dung from various large mammals (cows, donkeys and horses), and from dog, cat, rodent and human feces (4). C.difficile produces at least three potential virulence factors from which Toxin A and Toxin B are thought to be the most important in the pathogenesis of C.difficile associated disease (5).
Toxin A is an enterotoxin which seems to interfere with the cytoskeleton of the intestinal epithelial cells rendering them non functional while Toxin B is a cytotoxin that induces strong cytopathic effects in tissue cultures cell lines (6) which started to appear in late 1990’s (7).
Since not all strains of Clostridium difficile produce toxins and approx. 2% of healthy adults as well as up to 50% of children younger than 2 years can be colonized with Clostridium difficile, the detection of the toxins (Toxin A and Toxin B) in stool samples of patients with diarrhea is more significant than culturing the bacteria.
Keul-o-test Clostridium Difficile B is a fast, simple and highly sensitive test for the rapid and reliable detection of C.difficile Toxin B antigen in the feces.
The method employs a unique combination of monoclonal dye conjugate and polyclonal solid phase antibodies to selectively identify Toxin B with a high degree of sensitivity and specificity.
After collection in a tube containing extraction solution, the sample is dissolved and a few drops of this extract are added to the sample well of the reaction device.
As the test sample flows through the absorbent device, the labelled antibody-dye conjugate binds to the Toxin B antigen (when present in the sample), forming an antibody antigen complex.
This complex binds to the polyclonal antibody in the positive reaction zone, producing a rose-pink coloured band.
In the absence of Toxin B, there is no line in the positive reaction zone. The reaction mixture continues flowing through the absorbent device, past the positive reaction zone and control zone.
Unbound conjugate binds to the reagent in the control zone, producing a rose-pink coloured band demonstrating that the reagents are functioning correctly.
II. Keul-o-test Clostridium Difficile B KIT COMPONENTS
Each kit contains everything needed to perform the tests.
- Keul-o-test Clostridium Difficile B devices
- Disposable plastic pipettes
- Plastic tubes filled with 2 mL extraction solution
- Sample applicators
- Instructions leaflet
III. STORAGE AND STABILITY
- All Keul-o-test Clostridium Difficile B is to be stored at room temperature (+2°C to +30°C) in the sealed pouch.
- Do not freeze the test kit.
- Keul-o-test Clostridium Difficile B is stable until the expiry date stated on the package label.
IV. PRECAUTIONS
- This test is designed for in vitro and professional use only.
- Read carefully instructions before using the test.
- Do not use after the expiry date shown on the package label.
- All reagents and materials coming in contact with potential infectious specimens must be treated with appropriate disinfectants or autoclaved at 121°C for at least one hour.
- Do not use a test from a damaged protective wrapper.
V. SPECIMEN COLLECTION AND PREPARATION
1) Preliminary notes
Stool specimen should be collected as soon as possible after onset of symptoms.
Diluted samples may be stored at +2°C to +8°C for 3 days without interference with assay performance.
For long term storage of undiluted specimens, storage at -20 °C or colder is recommended. Repeated freezing and thawing of samples is not recommended and may cause erroneous results.
Caution !
Do not collect specimens in containers having media, preservatives, animal serum or detergents as any of these may interfere with the test.
2) Procedure
a) Write the name of the patient on the plastic tube containing the extraction solution.
b) Open the tube and, using the sample applicator, transfer a stool sample (volume of a pea) into the tube, in case of solid stool.
If the stool is liquid, transfer 200 µl of liquid into the tube.
c) Tighten the cap and mix the stool sample and the diluent by shaking well until the sample is dissolved.
d) Let the tube stand long enough for the large particles to settle to the bottom of the tube. Alternatively, centrifuge the tube at 500-1,000 RPM for 1 minute.
VI. TEST PROCEDURE
- Bring all reagents at room temperature.
- Remove the test device from the pouch by tearing along the split.
- Open the plastic tube containing the extracted sample.
- Using the plastic pipette, add 6 drops (200 µl) of the extracted solution into the sample well.
- Read the results of the test 15 minutes after addition of the sample to the device.
VII. INTERPRETATION OF RESULTS
|
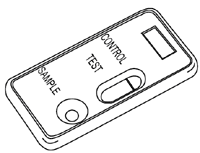
A. Negative
Only one coloured band appears in the control zone. No band is visible in the test zone. The sample does not contain C.difficile Toxin B.
|
|
|
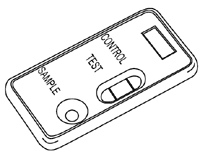
B. Positive
In addition to the control band, a clearly distinguishable band also appears in the test zone. The sample contains C.difficile Toxin B.
|
|
C. Inconclusive
If there is no distinct coloured band visible in both the test and control zones, the test is inconclusive. Repeat the test.
VIII. PERFORMANCES CHARACTERISTICS
a) Analytical sensitivity
The sensitivity of the test has been evaluated using a range of diluted solutions prepared from a commercially available purified C.D. Toxin B antigen. Under these conditions, the detection limit of the test has been found to be 5 ng/mL.
b) Specificity
A purified C.D.Toxin A antigen was used to determine the specificity of the test.
Keul-o-test Clostridium Difficile B showed consistently negative results up to 500 ng/mL Toxin A.
c) Reproducibility
1- Intra-assay
Within run precision was determined by using 3 replicates of 4 negative and positive specimens. The negative and positive values were correctly identified 100% of time.
2- Inter-assay
Between run precision was determined by using the same 4 negative and positive specimens with 3 different lots of reaction devices. Again, the negative and positive values were correctly identified 100% of time.
IX. LIMITATIONS
- Keul-o-test Clostridium Difficile B is specifically designed to detect Toxin B antigen in the stool samples.
- As for any diagnostic procedure, the physician should confirm the data obtained using this test by other clinical methods.
- A negative result does generally not exclude a C.difficile infection. It can be caused by proteolytic digestion of the toxins due to improper specimen storage. If a reasonable suspicion of an infection exists, another stool specimen should be investigated.
- A positive result does not exclude the presence of other pathogens.
- Test and control lines colours may slightly change depending on the stool sample aspect. For example dark green lines (instead of pink lines) have been reported when assaying greenish or darkish stool samples. This stool coloration appears in case of treatment of iron deficiency with ferrous fumarate. The test result should be interpreted as usual, i.e. two lines for a positive result and one line for a negative result.
X. BIBLIOGRAPHY
- Lierly D.M, H.C. Krivan, and T.D. Wilkins. 1988. Clostridium difficile : its disease and toxins. Clin. Microbiol. Rev. Vol.1 (1) :1-18.
- Mulligan. M.E., L.R. Peterson, R.Y.Y. Kwok, C.R. Clabots, and D.N. Gerding. 1988. Immunoblots and plasmid fingerprints compared with serotyping and polyacrylamide gel electrophoresis for typing Clostridium difficile. J. Clin. Microbiol. 26 (1) : 41-31.
- Gilligan P.H., L.R. McCarthy, and V.M. Genta. 1981. Relative frequency of Clostridium difficile in patients with diarrheal disease. J. Clin. Microbiol. 14 : 26-31.
- George. W.L. 1989. Antimicrobial agent-associated diarrhea and colitis, p661-678.
- Sullivan N.M., S. Pellett, and T.D. Wilkins. 1982. Purification and characterization of toxins A and B of Clostridium difficile. Infect. and Immun. 35 (3) : 1032-1040.
- Lyerly. D.M., D.E. Lockwood, S.H. Richardson, and T.D. Wilkins. 1982. Biological activities of toxins A and B of Clostridium difficile. Infect. Immun. Vol.35 (3) : 1147-1150.
- Wilkins T., Lyerly D.M.
Clostridium difficile testing : after 20 years, still challenging. J. Clin. Microbiol. 2003. Vol. 41 : 531-534.
Qualitätssicherung und Vorkommnisse
Sollten Sie den Eindruck eines Qualitätsmangels haben oder unklare oder ihrerseits falsch-positive oder falsch-negative Ergebnisse erhalten, bitten wir Sie, die betreffende Patientenprobe zurückzustellen und für einen Abruf für uns bereitzuhalten.
Bitte informieren Sie uns umgehend. Sie helfen uns dadurch die Sicherheit der Produkte und damit die Qualität zu gewährleisten.
 |
Hersteller: |

Günter Keul GmbH
Von-Langen-Weg 10
D-48565 Steinfurt
Tel.: 02551/2097 Fax.: 02551/80883
Web: www.keul.de
|
|
|