Keul-o-test
M. pneumonia IgM
KGST401
DIMDI Reg.-Nr.: DE/CA22/1116-259-IVD
Iimmunochromatographic rapid test for qualitative detection of antibodies against mycoplasma pneumoniae in serum/plasma
INTRODUCTION
The Keul-o-test M. pneumonia IgM is a chromatographic immunoassay for the rapid qualitative determination of IgM antibody to mycoplasma pneumonia in human serum or plasma.
Mycoplasma pneumoniae is the smallest and simplest self-replicating bacteria, which lacks a peptidoglycan cell wall, like all Mollicutes. Mycoplasma pneumoniae is a common cause of community-acquired pneumonia (CAP), and the disease usually has a prolonged, gradual onset.
SUMMARY AND PRINCIPLE OF THE TEST
Keul-o-test M. pneumonia IgM is a chromatographic immunoassay for the rapid qualitative determination of human anti-MP in serum. The membrane is pre-coated with recombinant MP P1 antigen on the test line region and goat anti-mouse IgG on the control band region. During testing, the serum specimen is allowed to react with the colloidal gold particles, which have been coated with mouse anti-human IgM. The mixture then moves laterally on the membrane by capillary action. For a positive result, a pink colored band with a specific MP-antibody-mouse anti-IgM-colloidal gold particle complex will form on the test line region. Absence of a colored line in the test line region indicates a negative result. To serve as a procedural control, a pink colored band at the control region will always appear regardless the presence of MP IgM.
REAGENTS AND MATERIALS
- Keul-o-test M. pneumonia IgM 25
- Sample Diluent 15mL
- Instruction for use 1
PRECAUTION FOR USERS
- For in-vitro diagnostic use only.
- Handle all specimens as if they contain infectious agents. When the assay procedure is completed, dispose of specimens carefully after autoclaving for at least one hour. Alternatively, treat with a 0.5 or 1% solution of sodium hypochlorite for one hour before disposal.
- Wear protective clothing (laboratory coats and disposable gloves) when assaying samples.
- Do not eat, drink or smoke in areas where specimens and kit reagents are handled.
- Avoid contact between hands and eyes or nose during specimen collection and testing.
SPECIMEN COLLECTION AND PREPARATION
The Keul-o-test M. pneumonia IgM is performed on human serum, plasma or reclassified plasma. Patient samples are best performed if tested immediately. Specimens should be refrigerated at 2-8°C, immediately following collection, for up to 3 days. If testing within 3 days is not possible, the specimens should be frozen (-20°C). If specimens are to be shipped, they should be packed in compliance with regulations covering the transportation of etiologic agents. Specimens containing precipitate may give inconsistent results. Such specimens should be clarified prior to assay.
STORAGE OF TEST KIT
The Keul-o-test M. pneumonia IgM Device can be stored at any temperature between 2-30°C. Do not freeze. The stability of the kit under these storage conditions is 24 months. Use up the reagents as soon as possible after the kit is unpacked.
TEST PROCEDURE
- When you are ready to begin testing, open the sealed pouch by tearing along the notch. Remove the test from the pouch.
- Draw 10ul serum sample into the pipette, and dispense it into the sample well on the cassette.
- Add 2-3 drops Sample Diluent into the sample well.
- Wait 10 minutes and read results. Do not read results after 15 minutes.
INTERPRETATION OF RESULTS
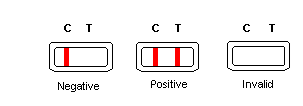
Negative: Only one colored band appears on the control region. No apparent band on the test region.
Positive: In addition to a pink colored control band, a distinct pink colored band will also appear in the test region.
Invalid: A total absence of color in both regions is an indication of procedure error and/or that test reagent deterioration has occurred.

LIMITATIONS OF THE ASSAY
- Keul-o-test M. pneumonia IgM Device is limited to the detection of human IgM antbody against MP in serum or plasma.
- As with all diagnostic tests, a definitive clinical diagnosis should not be made only on the basis of a single test. A complete evaluation by physician is needed for a final diagnosis.
RELATED READING MATERIALS
- Hollinger, F., et al. J. Immunol. 107:1099 (1971).
- Lander, J., et al. New Eng. J. Med. 285 (1971).
- Poleski, H.F. & Olson, C. Am. J. Clin. Pathol. 56: 129 (1971).
Qualitätssicherung und Vorkommnisse
Sollten Sie den Eindruck eines Qualitätsmangels haben oder unklare oder ihrerseits falsch-positive oder falsch-negative Ergebnisse erhalten, bitten wir Sie, die betreffende Patientenprobe zurückzustellen und für einen Abruf für uns bereitzuhalten.
Bitte informieren Sie uns umgehend. Sie helfen uns dadurch die Sicherheit der Produkte und damit die Qualität zu gewährleisten.
 |
Hersteller: |

Günter Keul GmbH
Von-Langen-Weg 10
D-48565 Steinfurt
Tel.: 02551/2097 Fax.: 02551/80883
Web: www.keul.de |
|